Is Water a Good or Bad Conductor of Electricity?
Water is a fascinating substance that plays a vital role in our daily lives. One intriguing question that often arises is whether water is a good or bad conductor of electricity.
The answer to this question lies in the presence of impurities in water. In this article, we will explore the conductivity of water, its dependence on impurities and temperature, and the importance of electrical safety near water.
Table of Contents
Conductivity of Water
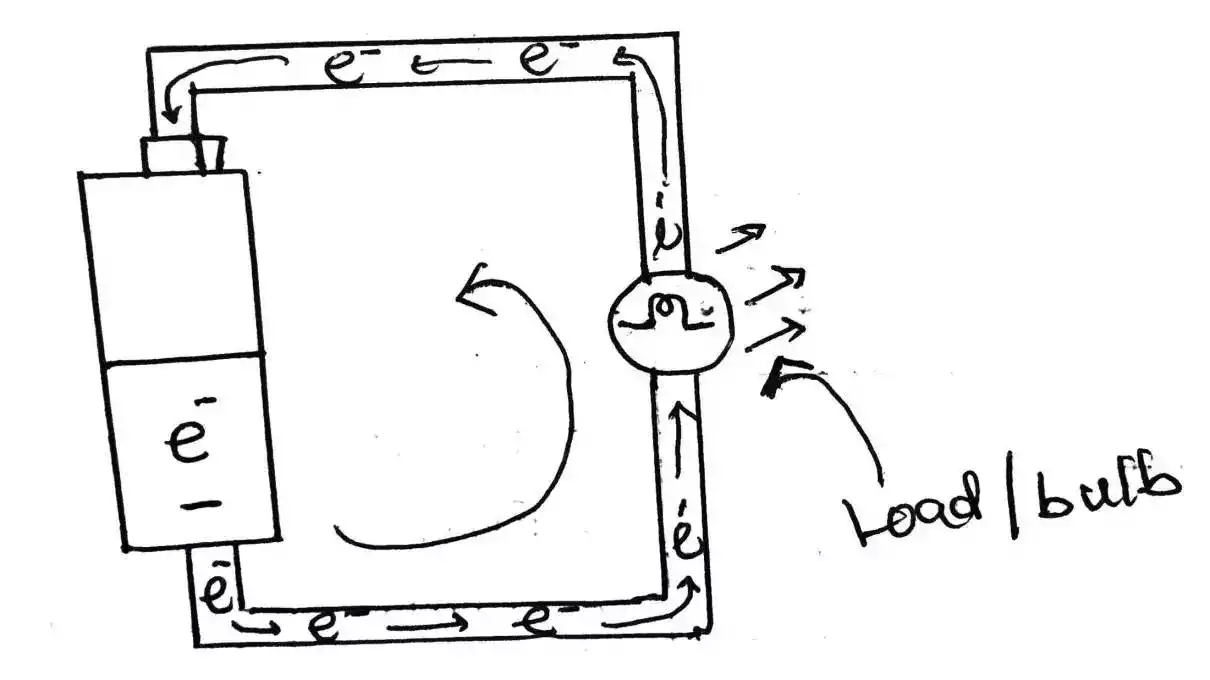
The conductivity of water refers to its ability to conduct an electric current. It is measured in siemens per meter (S/m).
Pure water, in its natural state, is a poor conductor of electricity. The conductivity of pure water is exceptionally low, typically around 10^-6 S/m. However, the story changes when impurities are introduced.
Impurities and Conductivity
When water contains dissolved impurities such as salts, it becomes a good conductor of electricity.
The impurities in water act as ions, which are electrically charged particles that can carry an electric current.
These ions facilitate the flow of electrons through the water, enabling conductivity. The presence of impurities increases the conductivity of water significantly.
Seawater, for example, which contains a high concentration of dissolved salts, has a much higher conductivity than pure water.
The conductivity of seawater typically ranges around 50 S/m, making it a relatively good conductor of electricity.
Temperature and Conductivity
Temperature also plays a role in the conductivity of water. As the temperature of water increases, its conductivity also increases.
The reason behind this relationship lies in the behavior of water molecules at higher temperatures.
When water molecules are heated, they gain energy and move more rapidly. This increased molecular motion makes it easier for the ions to move and carry an electric current. Consequently, the conductivity of water rises with temperature.
Electrical Safety Near Water
Understanding the conductivity of water is crucial when working with electricity near water sources.
The fact that water can conduct electricity highlights the importance of taking safety precautions.
If you come into contact with water that is conducting electricity, it can pose a serious electrocution risk.
To ensure electrical safety near water, consider the following tips:
- Never touch electrical equipment with wet hands: Moisture, including water, can enhance conductivity and increase the risk of electric shock. Always ensure your hands are dry when handling electrical equipment.
- Use a ground fault circuit interrupter (GFCI): Install GFCI outlets or use portable GFCI devices when working near water. GFCIs detect imbalances in electrical currents and quickly cut off power to prevent electric shock.
- Be aware of electrical outlets and switches in wet areas: When working in damp or wet locations, make sure you know the location of electrical outlets and switches. Keep them away from water sources and ensure they are properly sealed to prevent water ingress.
- Keep electrical cords away from water: Avoid running electrical cords or cables across wet surfaces or near water sources. Water can compromise the insulation of the cords and increase the risk of electrical shock.
By following these safety tips, you can minimize the risk of electrical shock when working with electricity near water.
Wrap Up
As a wrap up, pure water is a bad conductor of electricity, while water containing dissolved impurities, such as salts, is a good conductor.
The presence of impurities, combined with temperature, significantly affects water conductivity.
It is essential to exercise caution when working with electricity near water to avoid the risk of electrical shock.
By following proper safety measures and understanding the conductive properties of water, you can work safely in such environments.
Frequently Asked Questions (FAQs)
- Can pure water conduct electricity at all? Pure water is a poor conductor of electricity. It requires the presence of dissolved impurities, such as salts, to become a good conductor.
- Why does water conductivity increase with temperature? The increased temperature causes water molecules to move more quickly, facilitating the movement of ions and thus increasing the conductivity of water.
- How is water conductivity measured? Water conductivity is measured in siemens per meter (S/m) using specialized instruments called conductivity meters.
- What are some common impurities in water that increase its conductivity? Common impurities that increase water conductivity include salts, minerals, and other dissolved solids.
- Why is electrical safety important near water? Water’s ability to conduct electricity poses a significant risk of electric shock if you come into contact with water that is conducting an electric current.
- What is a ground fault circuit interrupter (GFCI)? A ground fault circuit interrupter (GFCI) is a safety device designed to quickly detect imbalances in electrical currents and cut off power to prevent electric shock.